Biomaterials in Application – One Technology, Endless Possibilities!
Bioprinting, also also referred to as biofabrication, has rapidly evolved into a transformative technology that unites additive manufacturing with biological materials. The core principle involves the layer-by-layer deposition of bioinks to construct precisely organized tissue structures. These bioinks typically consist of cells, biomaterials, and growth factors. Playing a pivotal role in regenerative medicine, bioprinting enables the creation of patient-specific tissues and organs for research, transplantation, and drug testing. The ultimate goal within the field is to fabricate fully functional organs suitable for human transplantation. However, significant challenges remain in replicating living, functional tissue structures at the organ level. Alongside the continuous advancement of biomaterials, manufacturing methods and process strategies must also be redefined and advanced.
When digital meets biological transformation
One promising approach lies in integrating production and biotechnology. By linking biological systems with digital and mechanical technologies, entirely new possibilities emerge. Change is no longer driven by a single innovation but by the convergence of multiple technologies that together create unique market breakthroughs. Within bioprinting, advancing process automation is essential. Selecting the right dosing technology is a key enabler of this progress, ensuring precision and reproducibility throughout the printing process.
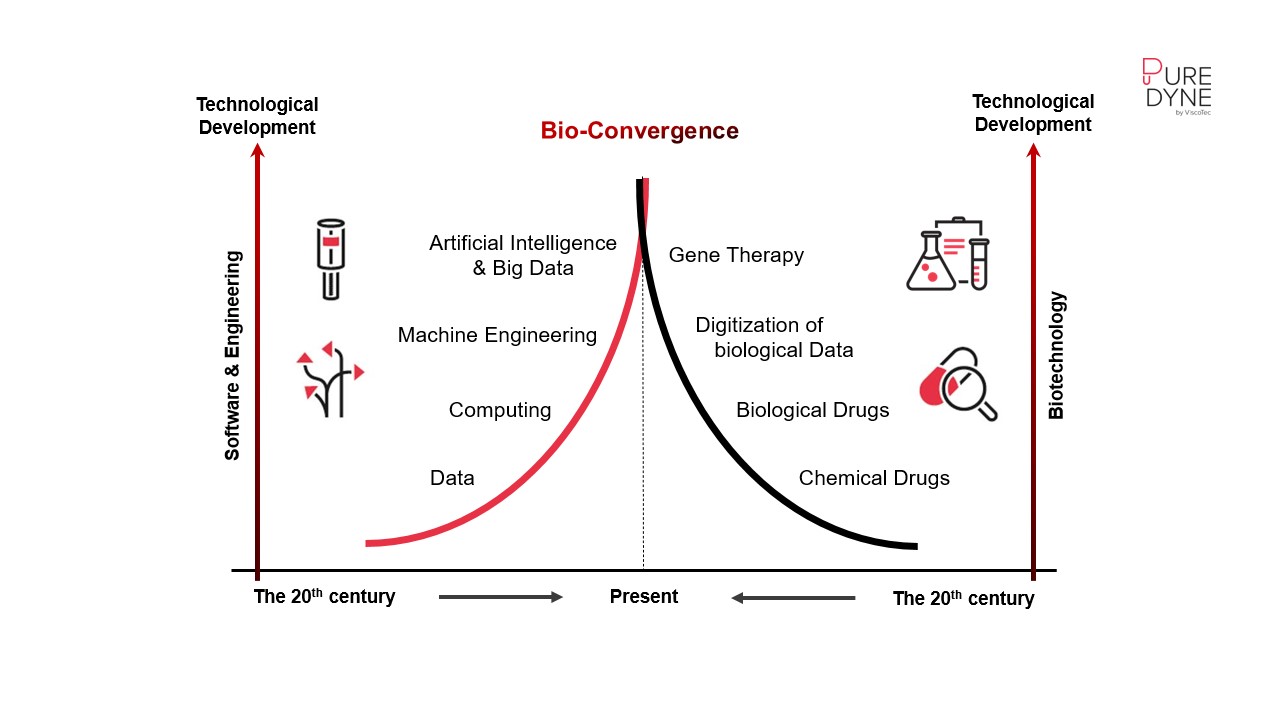
The following sections outline current possibilities, compare available technologies, and present automation solutions designed to optimize bioprinting performance.
State of the Art: Setting New Standards in Dispensing Technology
Alongside the development of high-performance bioinks, dispensing technology plays a critical role in achieving optimal printing outcomes.[1]. A variety of dispensing systems based on different physical principles are available on the market, which can generally be divided into two categories. The first includes technologies that use a nozzle as the outlet, while the second encompasses optical processes such as stereolithography and two-photon polymerization. The first classification primarily includes extrusion, inkjet, and laser-based processes.[2] This report focuses specifically on extrusion-based bioprinting, exploring its operating principles in greater detail and comparing its advantages and limitations.
Extrusion-based bioprinting is one of the most widely used 3D technologies in this field.[3] Renowned for its versatility, cost-efficiency, and ability to process a wide range of bioinks, this technology enables highly precise tissue fabrication. It is typically divided into three main types:
- Pneumatic extrusion
- Piston-driven extrusion
- Screw extrusion
In pneumatic extrusion, bioink is dispensed through a nozzle using controlled compressed air and deposited onto the substrate. This method is particularly suitable for materials with low to medium viscosity, such as soft hydrogels.[4], and can be easily automated. However, variations in material behavior and external factors, such as temperature or pressure fluctuations, can pose challenges for users and significantly affect key parameters like reproducibility.
Piston-driven extrusion uses a piston mechanism to precisely feed bioinks through the nozzle. This technology is particularly suitable for highly viscous materials and enables precise dosing and fast response times. However, mechanical stress can impair cell viability, while increased wear on components requires regular maintenance.[5]
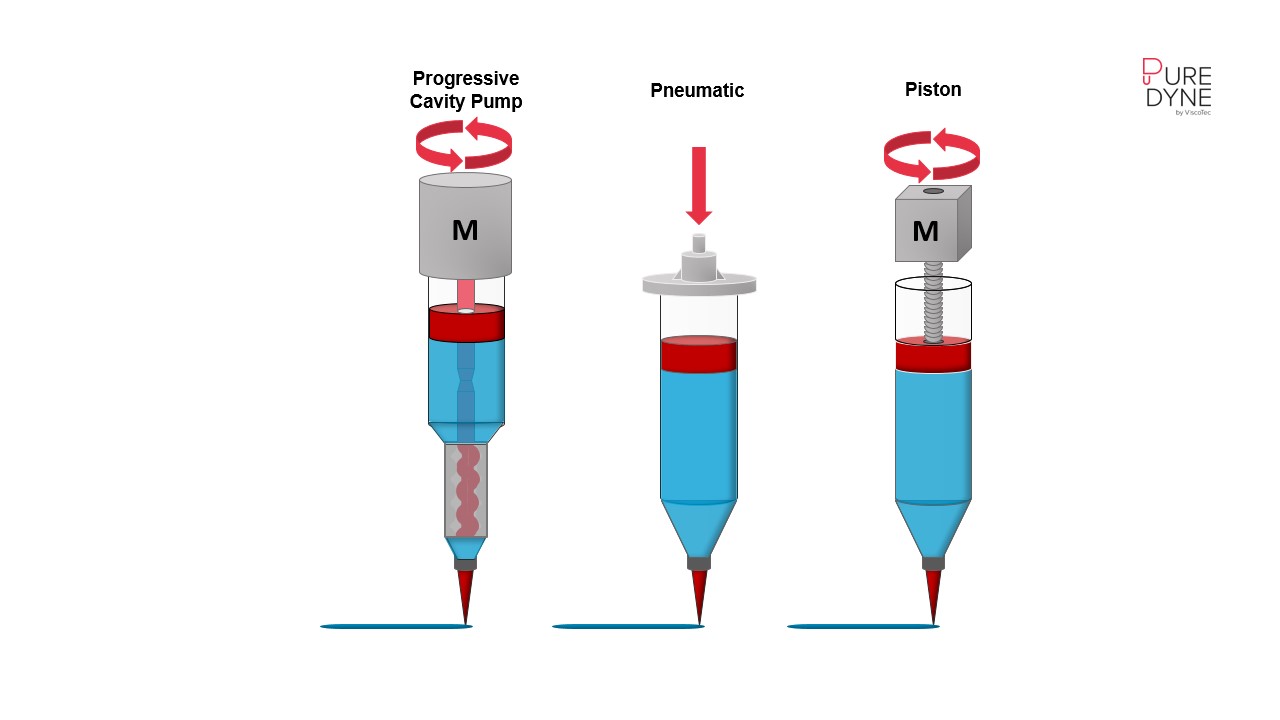
In the literature, screw extrusion is described as a process involving a spindle and a spindle valve.[6] The progressive cavity pump technology used at Puredyne belongs to the group of rotary positive displacement pumps and can be classified in this category in a broader sense. The main components of the technology are the precisely fitting rotor and stator system. The oscillating rotor conveys the materials in an existing stator. Conveying chambers are formed in which the biomaterials are transported from the inlet of the cartridge to the outlet side (to the needle) with hardly any pulsation and little shear force.[7] This enables the processing of low to high viscosity biomaterials.
Challenges in Developing the Optimal Cell Environment
The overarching goal of this research is to develop optimal scaffolds that support living cells. Accordingly, biomaterials are continuously refined and adapted to improve their performance. Extrusion-based technologies are preferred because they allow printing of hydrogels with a wide range of viscosities (up to 100,000 mPas) while maintaining high cell viability. The key challenge lies in fabricating complex structures that accurately replicate the properties of biological tissue.
The selection of an appropriate biomaterial is a critical factor that influences both the printability and the biological functionality of 3D-printed tissue constructs. Certain biomaterials, such as Pluronics, offer excellent rheological and mechanical properties that facilitate high-resolution printing, however, often lack the bioactivity necessary to support cell viability, proliferation, and tissue integration, making them suboptimal for long-term biological applications. Conversely, biologically favorable hydrogels like gelatin or gelatin methacrylate (GelMA) provide a supportive environment for cell encapsulation and growth, but they pose significant challenges during the printing[8] process. Issues such as high viscosity at room temperature, poor shape fidelity, and nozzle clogging can compromise the structural integrity and resolution of printed constructs. In addition to material properties, several process parameters play a vital role in determining the overall success of the bioprinting process. These include viscosity, extrusion pressure, printing speed, temperature control, needle diameter and geometry, as well as cross-linking methods. These variables must be finely tuned to ensure print fidelity and cell viability, often requiring iterative optimization for specific tissue engineering applications.
Progressive Cavity Pump Technology – High Degree of Automation
The challenges outlined above must be carefully considered when selecting appropriate dosing technology for bioprinting applications. This is where the Puredyne system provides significant advantages, offering process flexibility for a wide range of biomaterials in combination with 3D printing platforms. As previously described, current research is focused on the development of novel biomaterials. The objective is not to adapt materials to specific dispensing technologies, but rather to design materials optimized for the intended biological application, particularly those involving living cells. The Puredyne print head, utilizing an eccentric screw mechanism, enables this flexibility by allowing the precise and continuous extrusion of materials across a broad viscosity range — up to approximately 150,000 mPas — including those containing fillers, particles, or other additives[9].
With its emphasis on material-centric printing, the Puredyne system supports users in routine laboratory workflows, ensuring high precision and reproducibility in biomaterial deposition. The modular motor unit is engineered for adaptability and can be seamlessly integrated into standard 3D printers or programmable logic controllers (PLCs). The system’s broad compatibility makes it suitable for a wide range of applications, from bioprinting of human skin constructs to early-stage exploratory research, where flexibility in material handling is critical to success.
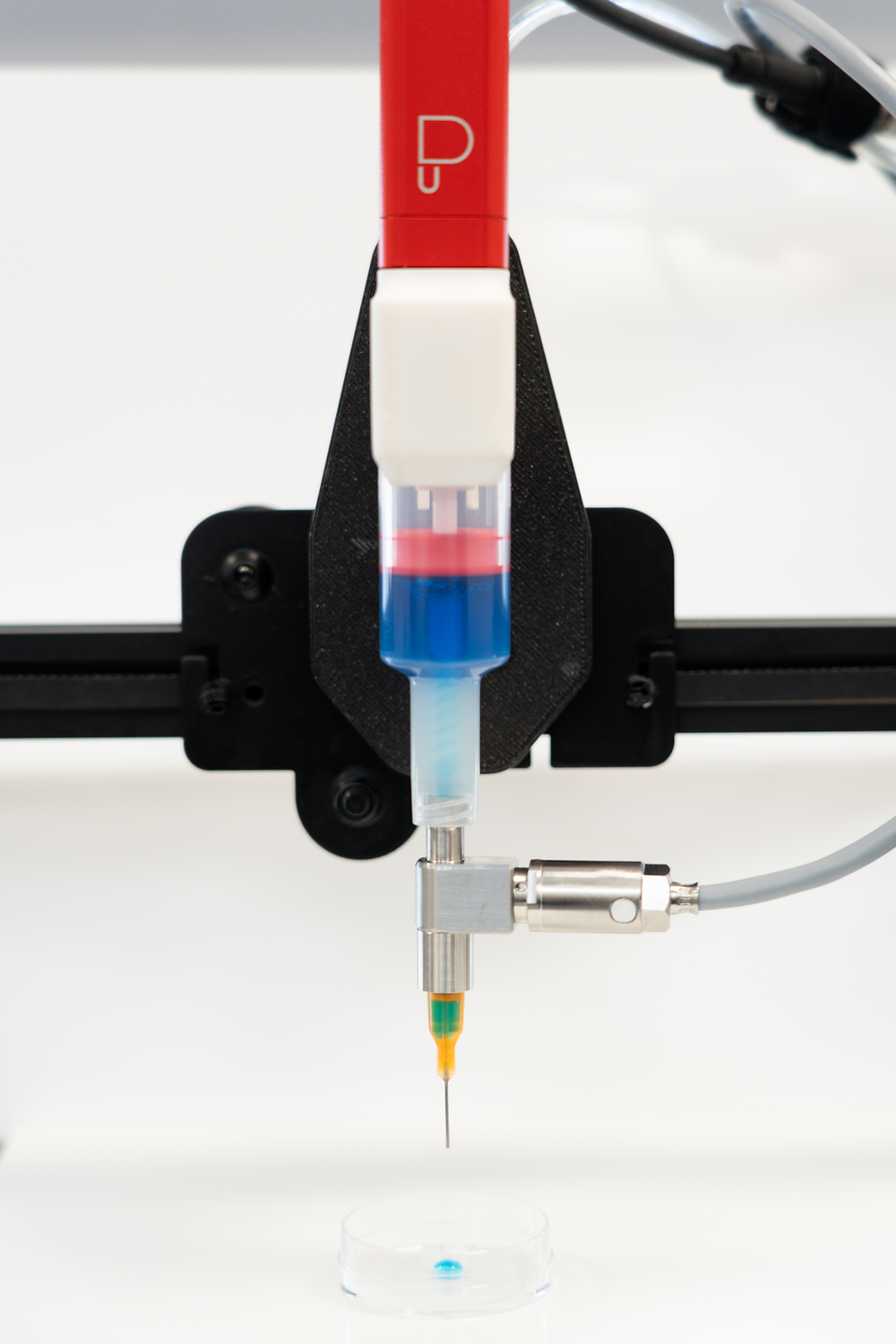
Another important factor influencing print quality and material behavior is the temperature input into the biomaterial. Puredyne’s cooling and heating unit makes it possible to ensure optimal survival conditions for the cells throughout the process, as temperatures can be set between 4 °C and 40 °C. This allows the flow behavior of the liquids to be positively influenced by heating or cooling them, ensuring a stable process with reproducible results, especially in fluctuating ambient temperatures. The heating and cooling unit can be easily connected to the Puredyne dosing head via a plug-in mechanism to form a pressure unit. The level of the medium can be conveniently checked by visual inspection as the unit’s housing is equipped with a viewing slot. The ability to adjust the temperature at the print head in both directions ensures that the application temperature of the bioink remains constant during dispensing. This enables stable and reproducible process parameters, which are essential for consistent printing results.
The integration of a pressure sensor enables real-time process control for the first time and significantly enhances the capabilities of the Puredyne dosing platform. This in-process control continuously measures the pressure of the dispensed biomaterial between the cartridge and the dosing needle, allowing users to monitor the entire bioprinting process in real time and identify potential risks, such as cell damage, at an early stage. Beyond immediate monitoring, the pressure sensor also lays the foundation for machine learning applications, enabling ongoing optimization of printing results.
The following sections outline current possibilities, compare available technologies, and present automation solutions designed to optimize bioprinting performance. Previously, process control in bioprinting relied largely on downstream optical inspection, which limited both accuracy and responsiveness. With this new capability, users are now equipped with the tools needed to make their bioprinting workflows safer, more controlled, and increasingly automated.
Outlook – Future-Proof Dosing Process
Bioprinting, as an emerging field within additive manufacturing (AM) technologies, presents both significant challenges and substantial potential. In recent years, there has been a marked increase in scientific publications, and importantly, the first bioprinting applications have progressed from the experimental phase into clinical trials. As the field continues to evolve, attention is now shifting beyond material development toward improving the efficiency and reliability of manufacturing processes.
Due to the complexity of achieving a precise and reliable dosing process in bioprinting, there is an increasing demand for technologically advanced systems combined with specialized dosing expertise. Puredyne addresses both needs by offering a comprehensive solution that integrates high-performance dosing technology with the flexibility required for a wide range of biomaterials and applications. Whether in manual operation for research environments or in automated production workflows, Puredyne systems provide the process stability, precision, and reproducibility essential for advancing bioprinting from the laboratory to clinical and industrial use.
Feel free to contact us at any time to take the next step in your process. www.puredyne.com
Sources:
[1] X.B. Chen, A. Fazel Anvari-Yazdi, X. Duan, A. Zimmerling, R. Gharraei, N.K. Sharma, S. Sweilem, L. Ning, (2023), Biomaterials / bioinks and extrusion bioprinting, Bioactive Materials, Volume 28, Pages 511-536, ISSN 2452-199X
[2] Santoni, S., Gugliandolo, S.G., Sponchioni, M. et al. (2022), 3D bioprinting: current status and trends – a guide to the literature and industrial practice. Bio-des. Manuf. 5, 14–42, https://doi.org/10.1007/s42242-021-00165-0
[3] I.T. Ozbolat, M. Hospodiuk, Current advances and future perspectives in extrusion-based bioprinting, Biomaterials (2016), https://doi.org/10.1016/ j.biomaterials.2015.10.076.
[4] Ozbolat, I. T. (2016). 3D Bioprinting: Fundamentals, Principles and Applications. Elsevier Inc.
[5] Kalyani Shinkar, Kawal Rhode (2022), Could 3D extrusion bioprinting serve to be a real alternative to organ transplantation in the future?, Annals of 3D Printed Medicine, Volume 7, 100066, https://doi.org/10.1016/j.stlm.2022.100066
[6] Lepowsky E, Muradoglu M, Tasoglu S (2018), Towards preserving post-printing cell viability and improving the resolution: Past, present, and future of 3D bioprinting theory (PDF). Bioprinting. 11: e00034. doi:10.1016/j.bprint.2018.e00034
[7] Sergis, Vasileios; Kelly, Daniel; Pramanick, Ankita; Britchfield, Graham; Mason, Karl; Daly, Andrew (2024). In-situ quality monitoring during embedded bioprinting using integrated microscopy and classical computer vision. figshare. Journal contribution. https://doi.org/10.6084/m9.figshare.28033469.v2
[8] Srikanthan Ramesh, Ola L.A. Harrysson, Prahalada K. Rao, Ali Tamayol, Denis R. Cormier, Yunbo Zhang, Iris V. Rivero (2021), Extrusion bioprinting: Recent progress, challenges, and future opportunities, Bioprinting, Volume 21
[9] Fisch P, Holub M, Zenobi-Wong M (2021), “Improved accuracy and precision of bioprinting through progressive cavity pump-controlled extrusion”. Biofabrication. 13 (1): 015012. doi:10.1088/1758-5090/abc39b
